
 Location: Ch 13: Electrostatics
Location: Ch 13: Electrostatics

 Location: Ch 13: Electrostatics
Location: Ch 13: Electrostatics
|
 |
ELECTROSTATICS
IN THIS CHAPTER:
FEATURES: |
 lectricity in one form or another underlies just about everything around
you. Itís in the lightning flashing outside right now. Itís in the spark
beneath your feet when you scuff a rug, and it holds atoms together. In
todayís times, it is important to have an understanding of how the basics
of electricity can be manipulated to give people a prosperity that was
unknown before the 1870s. This chapter is about electrostatics, or electricity
at rest. Electrostatics involve electric charges, the forces between them,
and their behavior in materials.
lectricity in one form or another underlies just about everything around
you. Itís in the lightning flashing outside right now. Itís in the spark
beneath your feet when you scuff a rug, and it holds atoms together. In
todayís times, it is important to have an understanding of how the basics
of electricity can be manipulated to give people a prosperity that was
unknown before the 1870s. This chapter is about electrostatics, or electricity
at rest. Electrostatics involve electric charges, the forces between them,
and their behavior in materials.
|
|
|
| 1. | Every atom has a positively charged nucleus surrounded by negatively charged electrons (except H+ molecules) |
| 2. | All electrons are identical; they all have the same mass and charge. |
| 3. | The nucleus is composed of protons and neutrons (except hydrogen). A proton has 1836 times the mass but its charge is the same as the electronís. Neutrons have a mass slightly greater than a proton. |
| 4. | Atoms usually have the same number of protons and electrons; they thus have no net charge. |
As mentioned earlier, gravity is a force that attracts you to the earth. Now imagine another force that is billions of times stronger, that could crush things to the size of a piece of paper. Suppose that there is also another repelling force that is also billions of times stronger than gravity. The two balance each other and have no noticeable effect. These are the electrical forces. Electrical forces arise from subatomic particles. The experiments by Ernest Rutherford and Niels Bohr proved that there was a positively charged nucleus surrounded by electrons. These nuclei attract and hold the electrons in orbit. At the same time, these electrons repel each other due to a property called charge. By convention, electrons are considered negatively charged and protons are positively charged. Neutrons have no charge are unaffected by magnetic charges. The fundamental rule at the base of all electrical phenomena is:
Electrons and protons have electric charge. In neutral atoms, there are as many electrons as protons, so there is no net charge. The total positive charge balances the total negative charge exactly. If an electron is removed from an atom, the atom is then positively charged. Charged atoms are ions. Positive ions have more protons than electrons, and negative ions have more electrons than protons. Ions have lost or gained electrons, respectively. You cannot change the charge of an atom by adding or removing protons; you would be drastically changing the atom and you would have a nuclear reaction!
Although the innermost electrons in an atom are bound very closely to the nucleus, the outermost electrons are held very loosely and can be easily torn away. The amount of energy required to tear an electron varies from material to material; a rubber rod holds its electrons more than a sample of fur. When you rub the two together, electrons transfer from the fur to the rod, and they both become charged. Thus, an object that has unequal numbers of electrons and protons is electrically charged. If there are more electrons than protons, the object is negatively charged. If it has fewer electrons than protons, then it is positively charged. Electrons are neither created nor destroyed; they are simply transferred from one material to another. In all such processes, the principle of conservation of charge applies. The creation and destruction of electrical forces has never been seen. Ions always have an excess or deficiency of whole numbers of electrons; electrons cannot be divided into fractions. Therefore, an atom cannot have a charge of 3.2 or 5.9.
Within the atomic nucleus, elementary particles called quarks carry charges of 1/3 or 2/3 the magnitude of the charge of the electron. Each nucleon is made of three quarks. Since quarks always exist in combinations of three and seem inseparable, the whole-number-multiple rule of electron charge holds for nuclear processes as well.
Newtonís Law of Gravitation states that the gravitational
force between two objects of mass m1 and m2 is proportional to the product
of the masses and inversely proportional to the square of the distances
between them. Thus, Newtonís Law of Gravitation states:  ,
where G is the universal gravitational constant, 6.67x10-11
,
where G is the universal gravitational constant, 6.67x10-11![]() .
The electrical force between any two objects obeys a similar inverse-square
relationship with distance. Charles Coulomb discovered this relationship
in the 18th century. Coulombís law states that for charged particles,
the force between the charges varies directly as the product of the charges
and inversely as the square of the distance between them. Thus, Coulombís
law states:
.
The electrical force between any two objects obeys a similar inverse-square
relationship with distance. Charles Coulomb discovered this relationship
in the 18th century. Coulombís law states that for charged particles,
the force between the charges varies directly as the product of the charges
and inversely as the square of the distance between them. Thus, Coulombís
law states:  ,
where k is the proportionality constant, 9.0×109
,
where k is the proportionality constant, 9.0×109 ![]() .
The SI unit of charge is the coulomb, abbreviated C. It turns out
that a charge of 1C is not the charge of 1 electron, but actually the charge
of 6.24×1018 electrons. This may seem like an enormous
amount of electrons, but it represents the amount of charge that passes
through a 100W light bulb in 1 second. Thus, Newtonís law for gravitation
is very similar to Coulombís law for electrical charges.
.
The SI unit of charge is the coulomb, abbreviated C. It turns out
that a charge of 1C is not the charge of 1 electron, but actually the charge
of 6.24×1018 electrons. This may seem like an enormous
amount of electrons, but it represents the amount of charge that passes
through a 100W light bulb in 1 second. Thus, Newtonís law for gravitation
is very similar to Coulombís law for electrical charges.
Although most electrical forces balance out for astronomical
and everyday objects, they donít always do this at the atomic level. The
electrons of one atom may attract the electrons of another atom, and thus
a bond is formed due to an electrical charge (a hydrogen bond?)
|
|
The hydrogen atom is extremely simple. Its nucleus is a
proton with 1.7×10-27kg mass, and an electron with a mass
of 9.1×10-31kg, at a distance of 5.3×10-11m.
To solve for the electrical force, substitute the appropriate values for
Coulombís law. Thus, 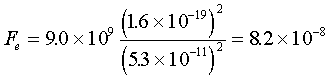 .
The gravitational force between them is: .
The gravitational force between them is:  .
To compare the two, just find a ratio between them. Thus, .
To compare the two, just find a ratio between them. Thus,  .
The electrical force, therefore, is 2.2×1039 times stronger
than the gravitational force. That means that the gravitational forces
that subatomic particles exert on each other are so much weaker than the
electrical force that gravitation can be completely ignored. (For our purposes
only; a truly accurate measurement would account for this.) .
The electrical force, therefore, is 2.2×1039 times stronger
than the gravitational force. That means that the gravitational forces
that subatomic particles exert on each other are so much weaker than the
electrical force that gravitation can be completely ignored. (For our purposes
only; a truly accurate measurement would account for this.) |
Whether or not a substance can be classified as a conductor or an insulator depends on how tightly the atoms of the substance hold their electrons. Some elements, such as germanium or silicon, are good insulators in their pure crystalline form, but become good conductors when 1 in 1 million atoms are replaced with impurities. These materials are semiconductors. These thin layers, when sandwiched together, make up transistors, ICs, and Pentium chips, such as the one this document is being written on. Other materials at very low temperatures (near 0K) acquire infinite conductivity. A few have even been shown to acquire this property at 100K. Explanations are still unknown but being researched. Now youíll have really fast computers (combined with bio-chips and the latest technology, things will soon be going at warp speed (literally!).
Unless you live in fantasyland, you have seen the electrical effects produced by friction. When we stroke a cat, scuff our shoes on a rug, or comb our hair in front of a mirror, electrical charges are changing with every snap, crackle and spark. In all these cases, electrons are being transferred by friction when one material rubs against another. Electrons can be transferred from one material to another by simply touching. When a charged rod is placed in contact with a neutral object, some of the charges on the rod will be transferred to the neutral object. If the object is a good conductor, the charge will spread to all parts of its surface because like charges repel. If it is a poor conductor, then you will have to touch it in several places to distribute the charge uniformly.
If we bring a charged object near a conducting surface, even without physical contact, electrons will move in the conducting surface. Consider two metal spheres, A and B. Normally, they are uncharged and touching, so they form a single noncharged conductor. When a negatively charged rod is held near sphere A, electrons in the metal are repelled by the rod, and excess negative charge has moved onto sphere B, leaving sphere A positively charged. The charge on the two spheres has been redistributed. A charge has been induced on the sphere. If the spheres are separated while the rod is still, present, the charge remains. If the rod is removed from the scene, the spheres will be charged equally and oppositely. They have been charged by induction. Since the charged rod never touches anything, it retains its initial charge.


Charging by induction occurs during thunderstorms like the one going on outside right now. The negatively charged bottoms of clouds induce a positive charge on the surface of the earth. When the clouds "bump" together, they discharge an enormous amount of energy to the ground. This is exactly the same phenomenon as when you comb your hair in front of a mirror! Benjamin Franklinís famous kite experiment proved that lightning was an electrical phenomenon, and he also discovered that charges flow readily between sharp objects, thus creating a lightning rod. Lightning rods prevent lightning by allowing charges between clouds and the ground to leak back and forth, thus preventing a charge buildup. If lightning does strike, it probably will hit the rod anyway, thus sparing any nearby buildings.
Charging by induction is not restricted to conductors. When a charged rod is brought near an insulator, there are no free electrons to migrate through the insulator. Instead, the charges rearrange themselves, and one side of the atom becomes more positive and the other side becomes more negative. Now the atom or molecule is electrically polarized. If the charged rod is negative, then the positive side of the atom or molecule is towards the rod, and the negative side is away from it. This process explains why electrically neutral bits of paper are attracted to a charged object. Molecules are polarized in the paper. Closeness wins, and the bits of paper are attracted towards each other. Sometimes they will they will cling to the charged object and then fly off. In this case, charging by contact has occurred; the paper bits acquire the same sign of charge as the charged object and are repelled.


If you rub an inflated balloon on your hair, you
will charge it. Place the balloon against the wall and it will stick because
the charge on the balloon induces an opposite surface charge on the wall.
Closeness wins yet again, for the charge on the balloon is slightly closer
to the opposite induced charge than the charge of the same sign.
 Many molecules, H2O, for example, are electrically polarized
in their normal states. The distribution of electrical charges is not exactly
even. There is a little more negative charge on one side of the molecule
than the other. Such molecules are electric dipoles (and can form hydrogen
bonds with one another).
Many molecules, H2O, for example, are electrically polarized
in their normal states. The distribution of electrical charges is not exactly
even. There is a little more negative charge on one side of the molecule
than the other. Such molecules are electric dipoles (and can form hydrogen
bonds with one another).
 In
summary, we know that objects are electrically charged in three ways:
In
summary, we know that objects are electrically charged in three ways:
In conclusion, all electrons have the same amount of negative charge; all protons have a positive charge equal in magnitude to the negative charge on the electron. Electrical forces arise because of the way that like charges repel and unlike charges attract. Throughout these processes, electric charge is neither created nor destroyed. According to Coulombís law, the electrical forces between two charged objects are proportional to the product of the charges and inversely proportional to the square of the distance between them. Electrons move easily in good conductors and poorly in good insulators. Objects become charged when electrons move onto or off of them. Charging by friction occurs when electrons are transferred by rubbing. Charging by contact occurs when electrons are transferred by direct contact. Charging by induction occurs in the presence of a charge without physical contact. Charge polarization occurs in insulators that are in the presence of a charged object.