
 Location: Ch 10: Introduction to Heat
Location: Ch 10: Introduction to Heat

 Location: Ch 10: Introduction to Heat
Location: Ch 10: Introduction to Heat
|
 |
INTRODUCTION TO HEAT
IN THIS CHAPTER:
|
 ll matter, no matter what phase it is in, is composed
of continually jiggling atoms or molecules. Because of this random motion,
it can be said that all matter has KE (and we haven’t gotten down to absolute
zero yet.) The average KE of these particles causes an effect we call heat.
To increase the average KE in a material, you can apply some energy, and
it warms up.
ll matter, no matter what phase it is in, is composed
of continually jiggling atoms or molecules. Because of this random motion,
it can be said that all matter has KE (and we haven’t gotten down to absolute
zero yet.) The average KE of these particles causes an effect we call heat.
To increase the average KE in a material, you can apply some energy, and
it warms up.
|
|
|
|
|
-459.4
|
-273
|
0
|
|
32
|
0
|
273
|
|
212
|
100
|
373
|
|
|
||
| Formula: | ||
| °Cà °F | 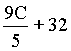 |
|
| °Cà K | ||
| Kà °F | 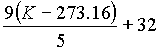 |
|
| Kà °C | ||
| °Fà °C | 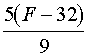 |
|
| °Fà K | 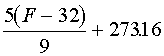 |
|
Conservation of Energy states that energy can neither be created nor destroyed. Although it appears that energy can indeed be created and destroyed, the energy is simply being converted from one form to another. In a Bunsen Burner, the spark converts chemical potential energy into thermal energy. However, energy is not always contained. Hot exhaust fumes mix into the atmosphere, heating it up a slight bit, but this hot air is still not hot enough to be useful.
If you touch a hot light bulb, energy will enter your hand from the light because the light is much hotter than your hand. However, if you touch a block of cast iron, energy will flow from your hand into the metal, and it seems to be cold. The direction of this spontaneous energy transfer is always from a hotter substance to a colder substance, even if the cooler object has more mass than the hotter one. These energy transfers are called heat. Heat, however, is not a property of matter. Heat cannot contain matter because it is only a transfer of energy. The energy that is transferred by the heat flow is called thermal energy. When heating takes place, the two objects transferring energy are said to be in thermal contact. Once the energy is transferred from the hot object to the warm object, the energy flow ceases, and they are in thermal equilibrium. To obtain an accurate measurement on a thermometer, we have to wait until it reaches thermal equilibrium with the substance being measured. We then know that thermometers should be small enough as to not alter the temperature of the substance being measured and that thermometers measure themselves!
In addition to the translational KE of jostling molecules, there is energy in other forms. There is rotational KE of molecules and KE due to internal movement of atoms. There is also potential energy due to the forces between molecules (hydrogen bonds?) The grand total of all these energies is called the internal energy (or thermal energy). Substances do not contain heat; they contain internal energy. When a substance absorbs or emits heat, any of these energies may change. In some cases, substances absorb heat without an increase of temperature — a phase change.
When a substance absorbs heat, the resulting temperature change depends on more than just the mass of the substance. The quantity of heat that boils a cupful of water may only raise the temperature of a pot of soup by a few degrees. To quantify heat, we must specify mass and the kind of substance The unit of heat is defined as the heat necessary
|
Energy
|
Description |
|
Translational
|
Energy of an object as it flies through something |
|
Rotational
|
Energy of molecules rotating in a solid |
|
Vibrational
|
Energy of vibrating molecules |
|
Potential
|
Energy that’s stored and held in readiness |
|
Thermal
|
The sum of all the above energies |
Different substances have different capacities for
storing internal energies. Water, for example, may take 10-15 minutes to
raise it from room temperature to boiling. Iron, however, would rise to
the same temperature in only 2 minutes. Specific materials require specific
quantities of heat to raise the temperature of a mass by a specific number
of degrees. Although an object may absorb lots of potential and rotational
energy, only translational KE changes temperature.
Since water absorbs a high quantity of heat for
rotations, internal vibrations and bonding, we say that water has the high
specific heat of 1![]() .
Although it may not seem like much, copper has a specific heat of 0.093
.
Although it may not seem like much, copper has a specific heat of 0.093![]() . Therefore, it could be said that water heats and cools slowly (in respect
to other materials).
. Therefore, it could be said that water heats and cools slowly (in respect
to other materials).
The high specific heat of water has many uses. Since a large amount of heat only results in a small temperature rise, water can be used as a cooling agent. Water also takes longer to cool, which means it can be used for foot-warmers. A large-scale example of this takes place in California. On the west coast, the winds generally blow air inland from the Pacific Ocean. Because of water’s high specific heat, the temperature of the water does not vary by much. Therefore, the water is cooler than the land during the summers, and the water is warmer than the land in the winters. Air passing overhead is cooled or warmed by the water and then blows over the land, keeping temperatures moderate. That’s why the Bay Area almost always has good weather. Since the air over the East Coast blows out to sea, the easterners suffer through cold, harsh winters and really hot summers.
When the temperature of a substance is increased, its molecules jiggle faster and move farther apart. This results in the substance expanding. When the temperature of a substance is decreased, the molecules jiggle slower and the substance contracts. With very few exceptions, all matter — solids, liquids and gases alike share this property. If concrete walkways and bridges were all built out of solid slabs, thermal expansion and contraction would soon weaken the concrete and cause it to crack. Therefore, walkways are always built in small sections with large gaps to expand in the hot summer heat. Likewise, bridges are built with expansion joints, sections of interlocking ‘teeth’ that allow for bridges to expand and contract without falling off the bridge (a bridge that doesn’t have such teeth would get no toll money at all!) Sometimes, thermal expansion can be put to use. A bimetallic strip is two pieces of different metals that are riveted or wielded together. These two pieces have vastly different rates of expansion, such as brass against iron. When the strip is heated or cooled, one side of the strip will become longer than the other side, causing the strip to bend. The movement of this strip could be used to operate a switch. The amount of expansion of a substance depends on its change in temperature. Rapid heating or cooling of glass may cause it to break. Liquids expand quite a bit in the heat. Gas overflowing on a hot day is a sure sign that the gas has expanded to the point where the tank cannot hold anymore of it. In most cases, the expansion rate for liquids is much greater than solids.
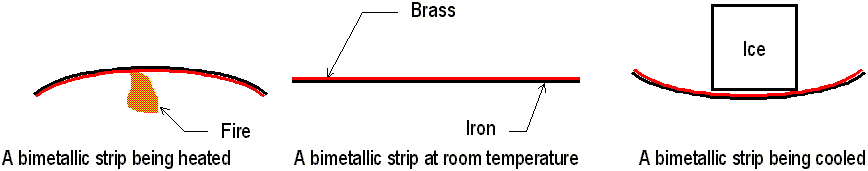
 A thermostat is a real-life use of a bimetallic strip. The bimetallic strip
is coiled into a spring. The back and forth bending of the strip opens
and closes a circuit to the heater In a cold room, the brass side contracts
faster, tilting a vial of mercury down towards two metal contacts, thus
completing the circuit to the heater. The heater comes on, and the bimetallic
strip warms up. The brass expands faster than the iron, and the strip tilts
the mercury vial back down, the circuit is broken, and the heater turns
off.
A thermostat is a real-life use of a bimetallic strip. The bimetallic strip
is coiled into a spring. The back and forth bending of the strip opens
and closes a circuit to the heater In a cold room, the brass side contracts
faster, tilting a vial of mercury down towards two metal contacts, thus
completing the circuit to the heater. The heater comes on, and the bimetallic
strip warms up. The brass expands faster than the iron, and the strip tilts
the mercury vial back down, the circuit is broken, and the heater turns
off.
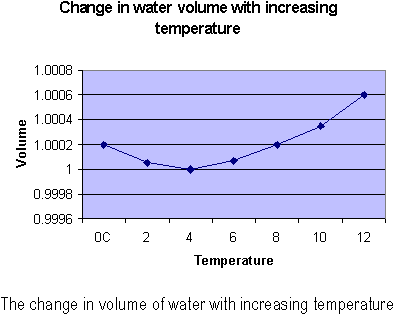
Almost all liquids will expand when heated. Ice-cold water, however, does the opposite! When ice is heated, it will contract until it reaches a temperature of about 4°C. Finally, the water begins to expand. Thus, water is most dense not as a solid, but at 4°C. Ice cannot be the densest form of water because huge glaciers manage to float above the water. This strange behavior of water happens because of the strange crystal structure of ice. Most solids are structured so that they are denser than in the liquid form. Ice, however, has open-structured crystals. These crystals result from the angular shape of water, plus the fact that the forces that bind H2O atoms together are stronger at some points. At 4°C, these crystal structures begin to break down; thus they can compact even more and become denser.
What would happen if water did not exhibit this bizarre behavior? Then the coldest water would settle to the bottom and ponds would freeze bottom-up, killing anything that was trapped in the pond. Fortunately, a different process occurs. Most of the cooling in the pond occurs at the surface. As the surface water is cooled, it becomes denser and sinks. The surface water will cool to 4°C, and it will sink. This loop repeats until all the water in the pond is 4°C. After that, the water continues to cool off until ice forms. Since ice is less dense than water, it floats to the top. Continued cooling of the pond results in even thicker ice, as the pond freezes downward. Large lakes may not freeze in winters because there will be a lot of water to cool. Because of water’s high specific heat and poor ability to conduct heat, the bottom of deep lakes in winter is a constant 4°C all winter long, and the fish can survive.
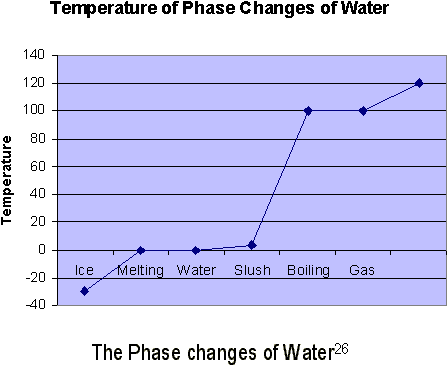
In conclusion, temperature is the measurement that tells how warm or cold something is. It is directly proportional to the average translational KE of the molecules within a gas. Heat is energy that transfers between two things due to a temperature difference (NOT a difference in thermal energy). Matter does not contain heat; it contains thermal energy. Thermal energy is the summation of all the energy contained in a substance. Specific heat is a measure of how much heat is required to raise the temperature of a given mass of a substance by a standard number of degrees. Water has a relatively high specific heat, which is useful for climate controls. Matter tends to expand when heated and to contract when cooled. Liquids usually expand more than solids, but gases expand far faster than any of them. Finally, water is highly unusual in that it contracts as it warms from 0°C to 4°C, and its solid form (ice) is less dense than its liquid form (water).