
 Location: Ch 11: Heat Transfer
Location: Ch 11: Heat Transfer

 Location: Ch 11: Heat Transfer
Location: Ch 11: Heat Transfer
|
 |
HEAT TRANSFER
IN THIS CHAPTER:
|
 pontaneous transfers of heat are always from warmer objects to cooler objects.
If several objects near each other have different temperatures, the warm
ones will cool and the cool ones will warm until all have a common temperature.
This equalization of temperatures is brought about in three ways: conduction,
convection, and radiation.
pontaneous transfers of heat are always from warmer objects to cooler objects.
If several objects near each other have different temperatures, the warm
ones will cool and the cool ones will warm until all have a common temperature.
This equalization of temperatures is brought about in three ways: conduction,
convection, and radiation.
If you hold one end of a metal rod in a flame, the rod will soon become too hot to hold. Heat is transferred through conduction. Conduction of heat only takes place between materials that are in direct contact. Materials that conduct heat well are known as heat conductors. Metals are the best conductors. Conduction results because of collisions between atoms or molecules and the actions of loose electrons. In the metal rod, the flame causes the atoms at the heated end to vibrate more rapidly. These atoms vibrate against neighboring atoms, which in turn do the same. The free electrons that can drift through the metal are made to jostle and transfer energy by colliding with atoms and other free electrons within the rod. Materials that have multitudes of these loose electrons are good conductors of heat and electricity. If you touch a block of metal and a block of wood, the metal seems colder even though both blocks are the same temperature! The metal feels colder because it is a better conductor; heat moves easily out of your hand and into the metal. Wood, however, is a poor conductor. Little heat moves into the wood, so you do not sense a cold object. An object that exhibits such characteristics is called an insulator. People's brains do not distinguish between two temperatures and two different conductivity rates, and things often seem cold when they are not.
Liquids and gases, in general, are good insulators. Air is a mixture of gases that conduct heat poorly. Porous materials that have small air spaces are very good insulators. The good insulating properties of materials such as wool, fur, feathers and double-paned windows are largely due to the air spaces they contain. Snowflakes imprison a lot of air inside the crystals and thus are good insulators. Snow slows the escape of heat from the earth’s surface, shielding Eskimo dwellings from the cold. Heat is energy and is tangible; cold is simply the absence of heat and therefore quite intangible. Only heat is transferred. Houses are not insulated to keep the cold out; they are insulated to keep the warm air in. Houses only become colder because the air flows out. No matter how good an insulator is, it still cannot prevent heat from going through it; insulators simply slow the rate at which heat penetrates.
Convection currents stirring the atmosphere produce winds. Some parts of the surface of the earth absorb heat more readily than other parts. The uneven absorption causes uneven heating of the air near the surface and creates convection currents. This strange phenomenon is often seen at the seashore. In the daytime, the shore warms faster than water. Air over the shore rises and cooler air from above the water takes its place. At night the process is reversed. As the shore cools off faster than water, air over the sea rises upward and air is drawn in from the shore. Rising warm air, like a balloon, expands because atmospheric pressure squeezes on it much less at higher altitudes. As the air expands, it cools, just like air warms as it is compressed. When the warm air expands, its molecules collide with slower moving molecules, and its own molecules slow down. Molecules generally collide with more molecules that are going slower but in the same direction. Therefore, the average speed of moleculess another application of Archimedes' Principle-the warmer fluid is buoyed upward by the denser surrounding fluid.
Heat from the sun is able to pass through the atmosphere and warm the surface of the earth. This heat does not pass by conduction, for air is not a good conductor. Nor does it pass through convection, for convection only starts after the earth is warm. Since conduction and convection are not possible in the near vacuum of space, the heat of the sun must be transmitted through another method—radiation. Any energy, including heat, which is transmitted by radiation is called radiant energy. Radiant energy is in the form of electromagnetic waves, which include radio, microwaves, infrared, visible light, ultraviolet, X and gamma rays. All objects continually emit radiant energy in a mixture of wavelengths. Objects at low temperatures emit long waves, just as long waves are produced when you shake a rope. High temperature objects emit waves of shorter wavelengths. Objects at room-temperature emit waves mostly at the long end of the infrared region. Shorter wavelength infrared waves absorbed by our skin produce the sensation of heat. Thus, when we speak of heat radiation, we are actually talking about infrared radiation. If an object is hot enough, some of the radiant energy it emits is in the range of visible light. At 500°C, an object begins to emit red light. Higher temperatures produce a yellowish flame. At about 1200°C, the object emits all light waves and it appears to be "white hot". Lights, fireplaces, and the sun emit both infrared and visible light, and they produce not only light but also heat.
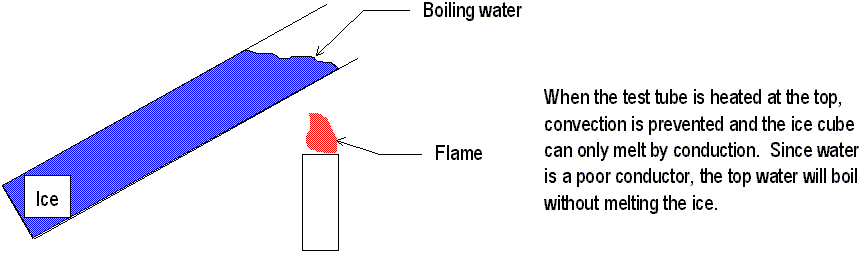
Absorption and reflection are complete opposites of each other. Therefore, a good absorber of radiant energy does not reflect much. Conversely, a good reflector does not absorb much radiant energy. A perfect absorber, such as the pupil of the eye, absorbs all the radiant energy. If you drill a small hole into a large white box, the box still appears black because the radiant energy that enters is reflected until very little or no radiant energy makes it back out.
Good absorbers are also good emitters; poor absorbers are poor emitters. For example, a radio antenna designed for broad cast will also be a good receiver. Likewise, a poorly designed transmitter will not receive broadcasts very well. Strangely enough, if a good absorber were not a good emitter, then black objects could never come to thermal equilibrium with white objects. But objects in thermal contact do come to equilibrium, so a black object must emit as much energy as it absorbs. If you fill a black and a white container full of hot water, the black pitcher will cool off faster than the white one. The surface of the black pitcher is a better emitter. Coffee and tea will stay hot longer in a shiny mirror-like pot than in a blackened one. The outside temperature determines the role of a surface. If the surface is hotter than its surroundings, it will be a net absorber and warm up. Conversely, if a surface is colder than its surroundings, it will become a net absorber and cool off. Every surface, hot or cold, absorbs and emits radiant energy. If the surface absorbs more than it emits, then it is a net absorber. If the surface emits more than it absorbs, then it is a net emitter. On a sunny day, the surface of the earth is a net absorber. At night it is a net emitter. On a cloudless night the earth's surroundings are the frigid depths of space (only 3Kelvin!), where the clouds are not the surroundings.
 As previously stated, an object at a temperature different from its surroundings
will ultimately come to a common temperature with its surroundings. The
rate of cooling of an object depends on how much hotter the object is than
its surroundings. The temperature change of a hot apple pie will be much
greater if you put it into a freezer than if you left it on the table.
The rate of cooling of an object—whether by conduction, convection or by
radiation, is approximately proportional to the temperature difference
DT between the object and its surroundings. Thus, rate of cooling (r)»DT.
This is known as Newton’s Law of Cooling. This law also applies to heating.
As previously stated, an object at a temperature different from its surroundings
will ultimately come to a common temperature with its surroundings. The
rate of cooling of an object depends on how much hotter the object is than
its surroundings. The temperature change of a hot apple pie will be much
greater if you put it into a freezer than if you left it on the table.
The rate of cooling of an object—whether by conduction, convection or by
radiation, is approximately proportional to the temperature difference
DT between the object and its surroundings. Thus, rate of cooling (r)»DT.
This is known as Newton’s Law of Cooling. This law also applies to heating.
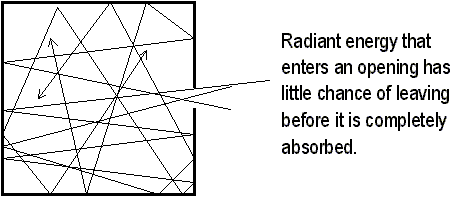
A car sitting outside on a bright and sunny day with the windows rolled up can get very hot inside. This is an example of the greenhouse effect, named after the temperature-raising effect in florists’ greenhouses. To understand the greenhouse effect, you need to know about two concepts. The first has already been stated in the previous sections. That concept is that all things radiate, and the wavelength of radiation depends on the temperature of the object emitting the radiation. High-temperature objects radiate short waves; low-temperature objects radiate long waves. The second concept involved in the greenhouse effect is that the transparency of objects depends on the wavelength of radiation. Air is transparent to both infrared and visible light, unless it contains excessive amounts of CO2 or water vapor. Glass is transparent to visible light but not to infrared.
The car gets hot for several reasons. Compared to the car, the temperature of the sun is extremely high. This means that the waves it radiates are very short (but they have a large amount of energy). These short waves pass easily through the atmosphere and into the car. After it enters the car, it hits the car’s interior and is absorbed. The interior of the car then warms up. The waves it emits, however, are too long to pass through the glass windows, and it just remains in the car. Unless the car catches on fire (and I sincerely hope this doesn’t happen), the energy just makes the car hotter and hotter until you open the window and let some of the energy escape into the atmosphere.
The same effect happens in the atmosphere of the earth. Since the atmosphere is transparent to solar radiation. The surface of the earth absorbs this energy and reemits some of this at a longer wavelength. Energy that the earth radiates is terrestrial radiation. Molecules of gas within the atmosphere absorb and reemit the terrestrial radiation back to Earth. So the long-wavelength waves that cannot escape warm the earth. If it were not for this, the earth would be quite cold! (-18°C to be exact.) During the last 500,000 years, the temperature of earth has varied between 19°C and 27°C. Our environmental concern is that the increased levels of CO2 may create a new thermal balance that will render the earth mostly uninhabitable. Greenhouses, as stated earlier, work in a process similar to the greenhouse effect in a car. A greenhouse’s heating is mainly due to the ability of glass to prevent convection currents from mixing with cool outside air.
In conclusion, heat transfer by conduction takes place within certain materials and from one material to another when they both touch each other. Metals are good conductors, while wood, cork, foam, liquids and gases are good insulators. Heat transfer by convection takes place by the movement of heated material itself. Convection occurs in all fluids (both liquids and gases). Winds result from convection currents that stir the atmosphere. Heat transfer by radiation takes place from everything to everything, even in a vacuum. Energy transmitted by radiation is called radiant energy. A good absorber of radiant energy reflects very little radiant energy, including visible light, and thus appears dark. Good absorbers of radiant energy are good emitters. According to Newton’s Law of Cooling, the rate of cooling (or warming) of an object is approximately proportional to the temperature difference between the object and its surroundings.