
 Location: Ch 12: Phase Changes
Location: Ch 12: Phase Changes

 Location: Ch 12: Phase Changes
Location: Ch 12: Phase Changes
|
 |
PHASE CHANGES
IN THIS CHAPTER:
FEATURES: |
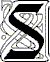 ince matter can exist in the three common phases, it must also be able
to change from one phase to another. Ice is the solid form of H2O.
Adding energy to the ice breaks down the rigid molecular structure and
the result is water. Add even more energy and the liquid changes to a gas,
namely steam. The phase of matter always depends upon the temperature and
pressure that is exerted on it. Changes of phase usually involve a transfer
of energy.
ince matter can exist in the three common phases, it must also be able
to change from one phase to another. Ice is the solid form of H2O.
Adding energy to the ice breaks down the rigid molecular structure and
the result is water. Add even more energy and the liquid changes to a gas,
namely steam. The phase of matter always depends upon the temperature and
pressure that is exerted on it. Changes of phase usually involve a transfer
of energy.
Water in an open container will eventually evaporate, or dry up. When this water disappears, it becomes water vapor in the air. Evaporation is the change of a liquid to a gas that occurs at the surface of the liquid. The temperature of matter is defined as the average KE of its molecules. Molecules in the liquid phase continuously move in all directions, bumping into each other at different speeds. Some of these molecules gain energy while others lose energy. Sometimes the molecules at the surface gain enough energy to break free of the liquid. They leave the liquid and fly into the space above the liquid. They now comprise a vapor, molecules in a gaseous state. Since the increased KE of the molecules comes from the molecules remaining in the liquid, the average KE of the liquid is lowered, and thus evaporation is a cooling process. A canteen keeps cool by evaporation when the cloth covering it is kept wet. As the water molecules leave the cloth, they decrease the temperature of the cloth. The cool cloth then cools the metal canteen through conduction, which also cools the water inside. When a person overheats, sweat glands produce perspiration that cools us and helps us to maintain a stable body temperature. Animals that lack sweat glands must pant or wallow in mud in order to simulate the effects of sweat glands.
The process opposite to evaporation is condensation—the changing of a gas to a liquid. The formation of droplets of water on the outside of a cold soda can is an example. Water vapor molecules collide with the slower moving molecules of the surface of the cold can. The vapor molecules give up so much KE that they cannot stay in the gaseous phase, and they condense. Condensation also occurs when liquids capture gas molecules. In their random motion, gas molecules may hit the surface of the liquid, lose KE, and then the attractive forces of the liquid traps the gas molecules inside the liquid. Condensation is a warming process. KE lost by condensing gas molecules warms the surface they strike. Therefore, a since steam is a vapor, a steam burn will do more damage than a hot water burn.
The air always contains water vapor. At any given temperature, there is a limit to the amount of it in the air. When this limit is reached, the air is said to be saturated. In weather reports, the relative humidity indicates how much water vapor is in the air, compared to the limit for that temperature. The relative humidity does not measure how much water vapor is in the air. A hot summer day with a low relative humidity may have more water vapor in the air than on a cold winter day with a high relative humidity. At a relative humidity of 100%, the air is completely saturated. More water vapor is required to saturated hot air than cool air. Warm tropical air can hold much more moisture than cold Arctic air. For saturation to occur, the water vapor in the air must be undergoing condensation. When slow-moving molecules collide, they sometimes stick together, or condense. These water molecules must be moving at a slow speed. At higher speeds they can bounce apart and then they remain gaseous. Although condensation in the air occurs more readily at low temperatures, it can also happen at high temperatures. There are always some molecules moving faster or slower than the average KE of the vapor. Even at high temperatures, there will be enough slow-moving molecules to cause condensation—provided there is enough water vapor present. Whatever the temperature, the slower-moving molecules are more likely to stick.
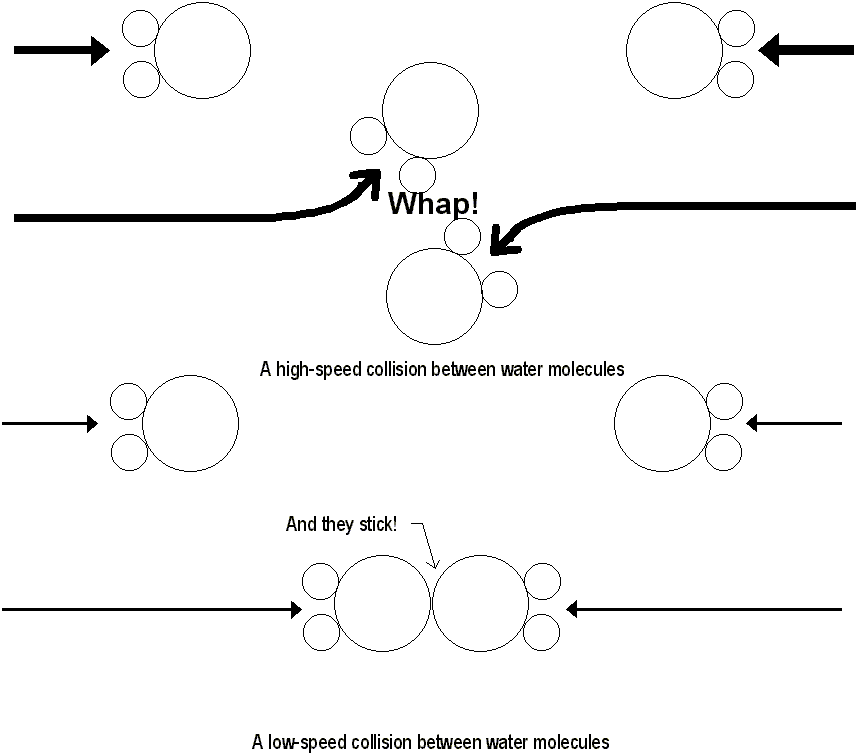
Most of these droplets of water are very small, about the size of dust particles. Since they are so small, they probably also have a low terminal velocity of about 1cm/s. The droplets of water seem to be held in suspension by a 1cm/s updraft. As the drops grow in size, their terminal velocity increases until it is greater than the updraft velocity, and they fall back to earth as rain.
If you emerge from a shower and step into a dry room, you feel cold because evaporation is occurring quickly. If you stay in the shower stall, you will not feel as cold. When you are in a moist environment, moisture from the air condenses on your skin and produces a warming effect that counteracts the effects of evaporation. If the rate of evaporation is the same as the rate of condensation, the temperature of your body will not change. If you leave a dish of water sitting on a table for many days, and the water level never goes up or down, it seems as if nothing were happening. However, the opposite is true; evaporation and condensation are occurring at the same rates. The molecules and energy that are leaving the liquid’s surface by evaporation are counteracted by as many molecules and as much energy returning by condensation. Thus the liquid is in equilibrium, since evaporation and condensation cancel each other out.
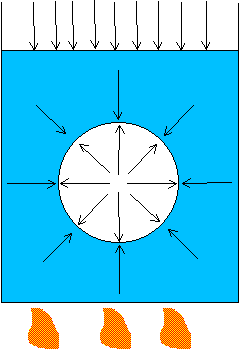 Evaporation and condensation normally take place at the same time. If evaporation
exceeds condensation, a liquid is cooled. If condensation exceeds evaporation,
a liquid is warmed. Most often heat is transferred from and to the surroundings,
so we do not notice cooling and warming effects due to evaporation and
condensation.
Evaporation and condensation normally take place at the same time. If evaporation
exceeds condensation, a liquid is cooled. If condensation exceeds evaporation,
a liquid is warmed. Most often heat is transferred from and to the surroundings,
so we do not notice cooling and warming effects due to evaporation and
condensation.
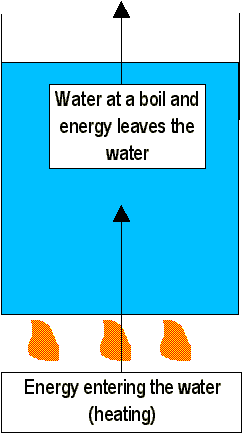
Evaporation has one flaw: it can only happen on the surface of water. However, a change of phase from liquid to gas can also take place beneath the surface of a liquid under the proper conditions. The gas that forms beneath the surface causes bubbles. These bubbles are buoyed to the surface where they escape into the surrounding air. This change of phase is called boiling. The pressure of the vapor within the bubbles in a boiling liquid must be great enough to resist the pressure of the surrounding water, or the water will collapse the bubbles.
As the atmospheric pressure is increased, the molecules within the vapor are required to move faster to exert increased pressure within the bubble in order to counteract the additional atmospheric pressure. So, increasing the pressure on the surface of a liquid raises the boiling point of the liquid. Conversely, lowering pressure (like at at high altitudes) decreases the boiling point. Thus, boiling depends not only on temperature but on pressure as well. However, food is cooked by water’s high temperature, not boiling itself. At high altitudes, water boils at a lower temperature. Since water boils at 95°C in Denver, Colorado, a "three-minute" boiled egg is runny. If the temperature of the boiling water were even lower, food would not cook at all.
 Pressure cookers use these principles to cook food quickly. A pressure
cooker has a tight-fitting lid that does not allow vapor to escape until
it reaches a certain pressure. As the evaporating vapor builds up inside
the cooker, pressure on the surface of the liquid is increased, which prevents
boiling. This raises the boiling point, and the food cooks faster.
Pressure cookers use these principles to cook food quickly. A pressure
cooker has a tight-fitting lid that does not allow vapor to escape until
it reaches a certain pressure. As the evaporating vapor builds up inside
the cooker, pressure on the surface of the liquid is increased, which prevents
boiling. This raises the boiling point, and the food cooks faster.
Boiling, like evaporation, is a cooling process. This is strange to some people because they associate boiling with heating. Heating, however, is one thing; boiling is another. When 100°C water boils, it is at thermal equilibrium (that is, it warms as fast as it cools.) If cooling did not take place, the water would continue to heat up, but none of it would turn to steam. A pressure cooker uses this principle to cook food quickly and efficiently because it prevents boiling, and thus prevents cooling.
When energy is continually withdrawn from a liquid,
molecular motion slows until the forces of attraction between them, causes
them to fuse. The molecules vibrate about fixed positions and form a solid.
This change in phase is called freezing. Strangely enough, if sugar or
salt is dissolved in the water, the freezing temperature will be lowered.
These foreign molecules or ions get in the way of the water molecules that
ordinarily would join into the hexagonal ice crystals. As ice crystals
form, this hindrance is intensified, and connection are more difficult.
Dissolving anything in water results in a harder to freeze mixture. Antifreeze
is mainly molecules that hinder freezing of coolant.
|
|
|
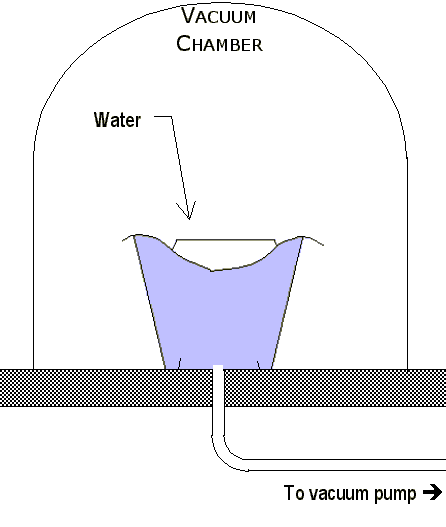 |
This is an interesting phenomenon, and one that seems impossible to somebody until they’ve seen it done. Suppose that a dish of water at room temperature is placed into a vacuum jar. If the pressure in the jar is slowly reduced by the vacuum pump, the water will start to boil. As previously stated, the boiling process cools the water. As the pressure inside is further reduced, more and more of the molecules boil away. Continued boiling reduces the temperature until the freezing point of 0°C is reached! If some drops of coffee are sprayed into such a chamber, they too will boil until they freeze. Even after they are frozen, the water molecules continue to evaporate until coffee crystals are left. This is how freeze-dried food is made. The low temperature of this process prevents the chemical structures from changing. When hot water is added to the coffee crystals, the original flavor of the coffee comes back. |
If you heat a solid sufficiently, it will melt and become a liquid. If you heat the liquid, it will vaporize and become a gas. Energy must be put into a substance to change its phase from solid to liquid to gas to plasma. Inversely, energy must be extracted from a substance to change its phase from plasma to gas to liquid to solid.
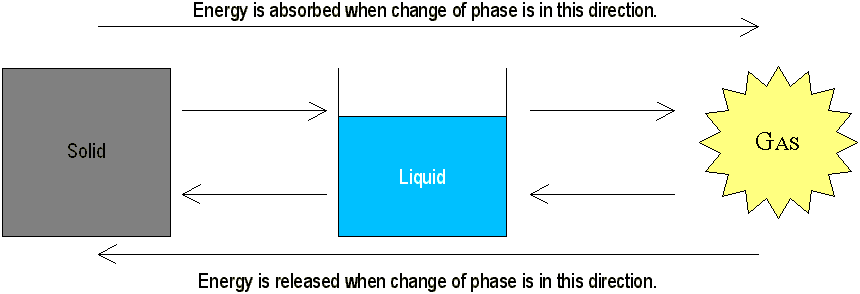
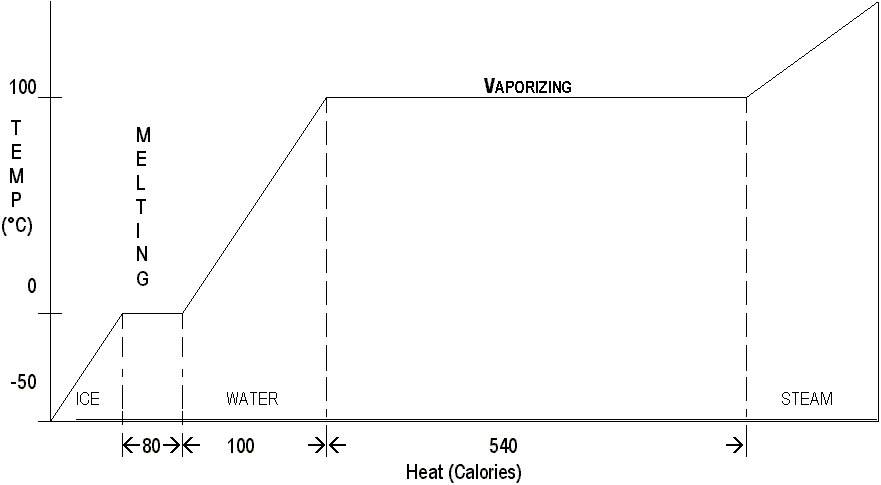
This process is reversible. When the molecules in a gram of steam condense to form boiling water, they release 540 calories of heat to the environment. When the water is cooled from 100°C to 0°C, 100 more are freed. When the ice fuses into ice, 80 calories are freed. The graph on the next page illustrates this.
The 540 calories required to vaporize a gram of water is a relatively large amount of energy—much more than to change a gram of ice from 0°C to 100°C. Although the molecules in steam and boiling water have the same average KE, steam has more PE because the molecules of steam are free of each other and are not held together in the liquid. Steam contains a vast amount of energy that can be released during condensation.
A refrigerator’s cooling cycle is an example of energy interchanges that occur with phase changes. The liquid is pumped into the evaporator where it is forced to evaporate and draw heat from the contents of the refrigerator. The gas is directed into the condenser out back, where heat is given off to the surrounding air and the gas condenses. The liquid is pumped back into the evaporator, and the cycle continues. Air conditioners work in exactly the same way. It is as if you were driving/living inside a refrigerator, except that air conditioners have a steady flow of air as to not asphyxiate the user. Although a heat pump and an air conditioner may seem opposites of each other, a heat pump is actually an air conditioner working in reverse: Heat is taken from the outside and "pumped" inside.
A way to test a clothes iron is to touch it with a finger. This is also a great way to burn a finger—unless it is moistened first. Energy that would go into burning the finger now goes into boiling the saliva. The energy converts the moisture into a vapor, which provides an insulating layer between the finger and the hot surface. Firewalkers also employ this technique to enable themselves to walk over hot coals without burning themselves. The primary thing they rely on is the low conductivity of heat from wood to the feet. A clumsy fire-walker who steps on a good conductor will be badly burned. A secondary factor is skin moisture. Perspiration decreases heat transfer to the feet. Much of the heat goes to boiling the sweat and not to the person’s feet.
In conclusion, a liquid changes phase and becomes a gas during evaporation. Evaporation is a cooling process. Conversely, a gas changes phase to a liquid during condensation. Condensation is a warming process. At the same relative humidity, there is more water vapor in warm air than in cold air. Clouds and fog form when air cools and is unable to contain as much water vapor. When evaporation and condensation occur simultaneously and at the same rate, the liquid is in equilibrium and there is no change in the volume of the liquid. A liquid is also in equilibrium when the surrounding air is saturated with vapor. In dry air, water evaporates much faster than it condenses; in humid air, it evaporates only slightly faster than it condenses. During boiling, a liquid changes phase at any place within the liquid and gas bubbles form. The boiling temperature of a liquid depends on the pressure at its surface. Boiling, like evaporation, is a cooling process. During freezing, a liquid changes phase to a solid. The freezing temperature of a liquid is lowered by adding other substances to it. Ice has a quasi-fluid layer of frozen water whose oxygen atoms vibrate 3 times the normal distance. Lastly, energy is given off or taken in during phase changes. While a substance changes phase, its temperature remains the same. Much more energy is given off when water condenses than when an equal mass of water freezes.